by Melissa Wenrich, Department of Geology, ASU
During January 1996, I and three other graduate students who work with the TES project at ASU ( Steve Ruff, Vicky Hamilton, and Kim Feely) had the unique and exciting opportunity to conduct some geological experiments using the actual TES instrument before it gets launched to Mars. We spent a week at Hughes Santa Barbara Remote Sensing (SBRS), working odd hours and late nights to collect infrared spectra of some of Earth's rock and mineral samples.
The TES is similar to the spectrometer in our laboratory at ASU, but it is much less bulky and is set up to detect infrared energy between wavelengths of 6 and 50 microns (a micron, or micrometer, is 1000 times smaller than a millimeter). Our lab spectrometer at ASU can only observe from 6 to 25 microns. The extra wavelengths that TES can "see" provide additional information about minerals that we can't get in our lab at ASU. The new knowledge as to what minerals look like in the 25 to 50 micron part of the spectrum will be very important when we get TES spectra from Mars in a few years. (continued after figure)
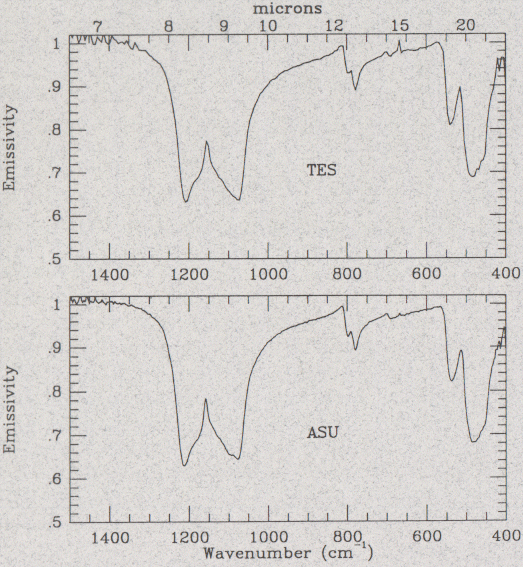
Thermal infrared spectra of the mineral, quartz. The top graph (spectrum) was obtained using the Mars Global Surveyor TES. The bottom spectrum is from our lab at ASU. The two spectra are nearly identical-- this is great news! Emissivity is the amount of infrared energy that was seen coming off the mineral. These spectra show the amount of energy emitted at each wavelength ("microns" along top axis) from about 6 to 25 microns. The dips or "valleys" in the spectrum are called absorption features. Figure courtesy Melissa Wenrich.
The TES experiments benefitted not only us graduate student scientists, but also the SBRS engineers who have put the TES together. For example, until we did our experiments with real minerals, the engineers had only tested TES's performance using a hot plate. The energy that is emitted from a hot plate is very different than what comes from a mineral. Minerals have distinct absorption features (parts of the spectrum where less energy is emitted-- the low areas in the quartz spectra seen in the Figure above). The absorption features are related to the way that a mineral vibrates when it is heated up. The TES engineers will be able to use the rock and mineral data we collected to help see if the TES is detecting the right information at each infrared wavelength in the spectrum.
The week we spent in the Santa Barbara, California, area was time well spent, with payoffs for both the scientists and engineers. Presently there is a discussion about having us go back to SBRS in March to analyze some more rocks and minerals and look at their infrared spectra. We hope we will get a chance to do this. The more we understand how TES sees these minerals, the better we will be able to use the instrument to learn about minerals on Mars. I and the other scientists involved with the spectrometer are looking forward to these additional tests; however, this science opportunity might disappear if the last few weeks before the TES is delivered to Lockheed-Martin in Denver are needed by the engineers to fine-tune the instrument (see Greg Mehall's report for more about this).
Once Mars Global Surveyor reaches Mars in late 1997, the TES will provide plenty of infrared spectra for us to interpret. We hope you will be following along with us as this all unfolds. Infrared data from Mars will be well worth the wait!
-- Melissa Wenrich is working on a Ph.D. in geology at ASU and is part of Phil Christensen's TES research group. She is studying the infrared spectra of carbonate minerals. Carbonates have carbon and oxygen in them and typically form in lakes and seas. An example of a carbonate mineral is calcite (calcium carbonate). Many antacid tablets you can buy in the store are made of carbonate minerals. She plans to finish her degree in 1997. She hopes to be an astronaut in the future. She enjoys hang-gliding, running, and hanging out with her dog, Oatmeal.